noble gas electron configuration|ru2+ electron configuration noble gas : Clark What would the noble gas electron configuration for an element look like if the element is a noble gas? Would it be. [Ar] or. [Ne]3s^23p^6 ? •. ( 16 votes) How to convert US dollars to Philippine pesos. 1 Input your amount. Simply type in the box how much you want to convert. 2 Choose your currencies. Click on the dropdown to select USD in the first dropdown as the currency that you want to convert and PHP in the second drop down as the currency you want to convert to.
noble gas electron configuration,Learn how to write a noble gas configuration for any atom using the Aufbau principle and the periodic table. See examples and a list of noble gas configurati.What would the noble gas electron configuration for an element look like if the element is a noble gas? Would it be. [Ar] or. [Ne]3s^23p^6 ? •. ( 16 votes)
This chemistry video explains how to write the electron configuration of an element using noble gas notation. Speed of Light, Frequency, Wavelength: • Speed of .
Start practicing—and saving your progress—now: https://www.khanacademy.org/science/c. How to write electron configurations for .

The chloride ions are chlorine atoms which have gained an electron and thus have the electronic structure 1 s2 2 s2 2 p6 3 s2 3 p6, the same as that of the noble-gas argon. .Learn how to write electron configurations for atoms, including noble gases, using the Aufbau principle and the Pauli exclusion principle. This web page is part of a free .
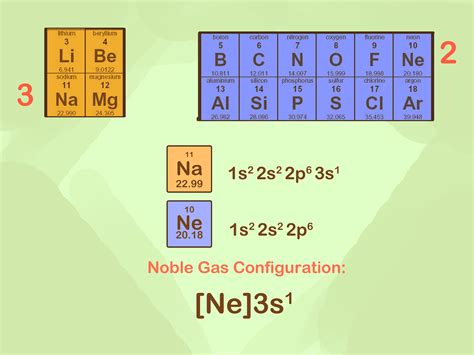
The distribution of electrons in respective atomic orbits of the noble gases is called noble gas configuration. The basis of any reaction taking place between chemicals is due to their impulsive behavior of achieving a . The noble gas configuration is written as the elemental symbol of the noble gas in the period before the element followed by the element’s remaining electrons. For instance, sodium’s full .The Noble gas shortcut electron configuration is a way of summarizing the information about the electrons of an atom which shows only the electrons most relevant for . Noble Gas Configuration. FlexBooks 2.0 >. CK-12 Chemistry for High School >. Noble Gas Configuration. Written by: Ck12 Science. Fact-checked by: The CK-12 Editorial Team. Last Modified: Jan 02, 2023.
Courses on Khan Academy are always 100% free. Start practicing—and saving your progress—now: https://www.khanacademy.org/science/chemistry/electronic-struct.Why is it possible to abbreviate electron configurations with a noble gas in the noble gas notation? 4. Identify the following elements: 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 6; . A noble gases with f electrons; (c) a . Electron configurations describe where electrons are located around the nucleus of an atom. For example, the electron configuration of lithium, . this can get quite complex. .The sodium ions are sodium atoms which have lost an electron, giving them the structure 1 s2 2 s2 2 p6, the same as that of the noble-gas neon. All electrons in both kinds of ions are paired. This page titled 6.5: Ions and Noble-Gas Electron Configurations is shared under a CC BY-NC-SA 4.0 license and was authored, remixed, and/or curated by Ed . The noble gas configuration is written as the elemental symbol of the noble gas in the period before the element followed by the element’s remaining electrons. For instance, sodium’s full configuration is 1s 2s 2 2 2p 6 3s 1 and neon’s is 1s 2s 2 2 2p 6. So, sodium’s noble gas configuration is [Ne]3s 1 . Part 1.

This chemistry video explains how to write the electron configuration of an element using noble gas notation.Speed of Light, Frequency, Wavelength: http.All of the electrons in the noble gas neon (atomic number 10) are paired, and all of the orbitals in the n = 1 and the n = 2 shells are filled. The electron configurations and orbital diagrams of these four elements are: The alkali metal sodium (atomic number 11) has one more electron than the neon atom.
To do this, the nearest noble gas that precedes the element in question is written first, and then the electron configuration is continued from that point forward. For example, the electron notation of phosphorus is 1s 2 2s 2 2p 6 3s 2 3p 3 .A Noble Gas is a group of elements that in their standard state have a filled electron cloud.. These elements are found in the 18th column of the periodic table and include Helium (He), Neon (Ne), Argon (Ar), Krypton (Kr), Xenon (Xe) and Radon (Rn). They are all odourless and colourless mono-atomic elements. Because these elements are already .They are helium, neon, argon, krypton, xenon, and radon. A noble gas configuration of an atom consists of the elemental symbol of the last noble gas prior to that atom, followed by the configuration of the remaining electrons. So for sodium, we make the substitution of [Ne] [ Ne] for the 1s22s22p6 1 s 2 2 s 2 2 p 6 part of the configuration. Noble Gas Notation. This is a way of writing an abbreviated electron configuration, with the noble gas substituting the beginning energy levels and orbital-filled shells. The idea is the .noble gas electron configuration Also, if you look at the table of electron configurations, it should be noted that Nb is an exception to the typical orbital filling rules) b) A similar procedure is followed for Pr, element number 59. Moving backward through the table, the nearest noble gas is Xe, and so we use the Xe kernel. Counting forward again, Cs and Ba correspond to 6s 2.All of the electrons in the noble gas neon (atomic number 10) are paired, and all of the orbitals in the n = 1 and the n = 2 shells are filled. The electron configurations and orbital diagrams of these four elements are: The alkali metal sodium (atomic number 11) has one more electron than the neon atom.This row concludes with the noble gas argon, which has the electron configuration [Ne]3s 2 3p 6, corresponding to a filled valence shell. Example \(\PageIndex{1}\): Electronic Configuration of Phosphorus . Ignore the inner orbitals (those that correspond to the electron configuration of the nearest noble gas) and write the valence electron .noble gas electron configuration ru2+ electron configuration noble gas The electron configuration and the orbital diagram are: Following hydrogen is the noble gas helium, which has an atomic number of 2. The helium atom contains two protons and two electrons. The first electron has the same four quantum numbers as the hydrogen atom electron ( n = 1, l = 0, ml = 0, ms = +1 2 m s = + 1 2 ).Video: Fe, Fe2+, and Fe3+ Electron Configuration Notation. In writing the electron configuration for Iron the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for Iron go in the 2s orbital. The next six electrons will go in the 2p orbital. The p orbital can hold up to six electrons. Shorthand notations make use of the fact that the noble gases have full outer electron shells, and some sources call it “noble gas notation” for this reason. Put the chemical symbol for the noble gas in front of the configuration in square brackets, and then write the configuration for any additional electrons in the standard way. Look at .
noble gas electron configuration|ru2+ electron configuration noble gas
PH0 · unpaired electrons calculator
PH1 · ru2+ electron configuration noble gas
PH2 · noble gas shorthand calculator
PH3 · noble gas electron configurations list
PH4 · noble gas electron configuration nobelium
PH5 · ground state electron configuration calculator
PH6 · full electron configuration for sodium
PH7 · electron configuration for every element
PH8 · Iba pa